Great Tips About How To Learn Electron Configuration

For example, the electron configuration.
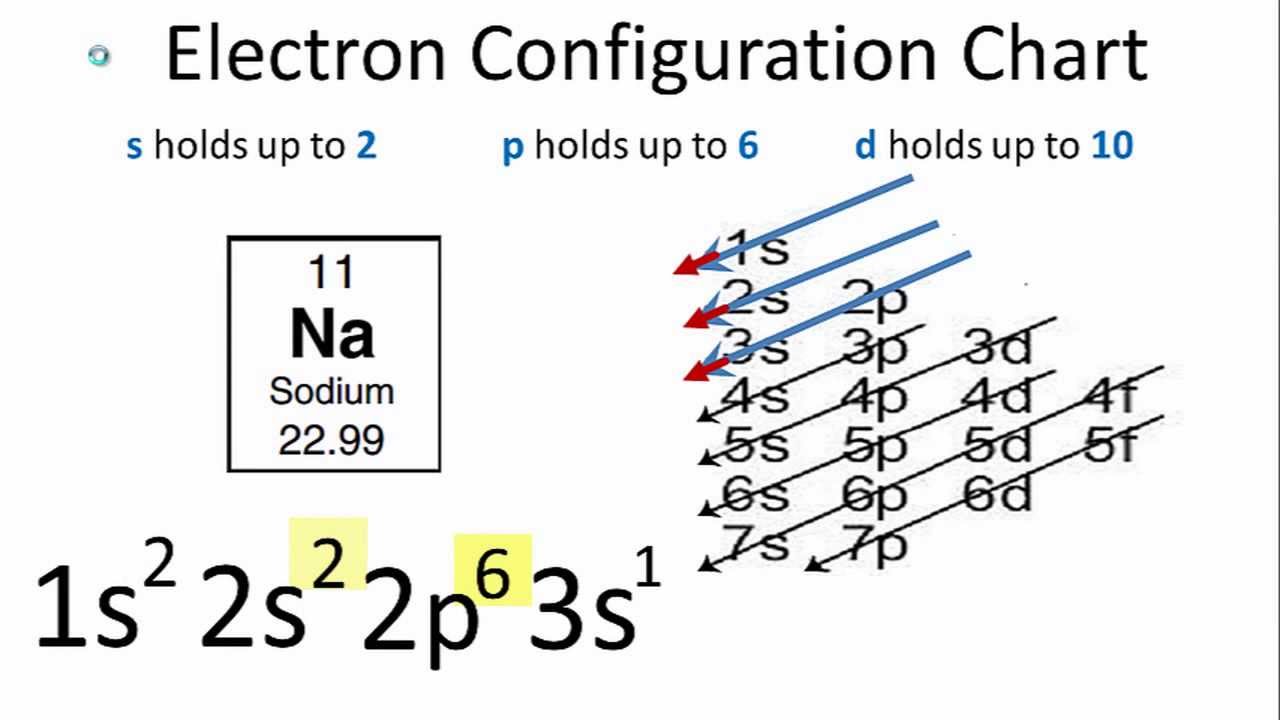
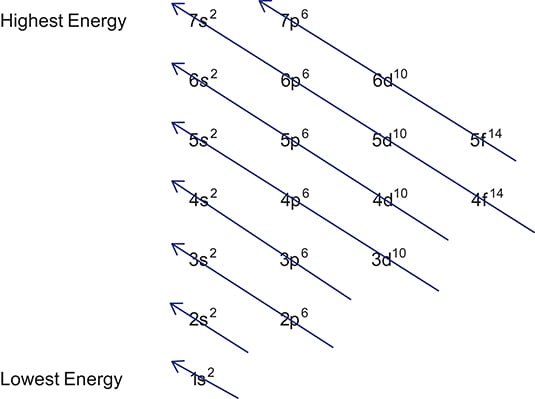
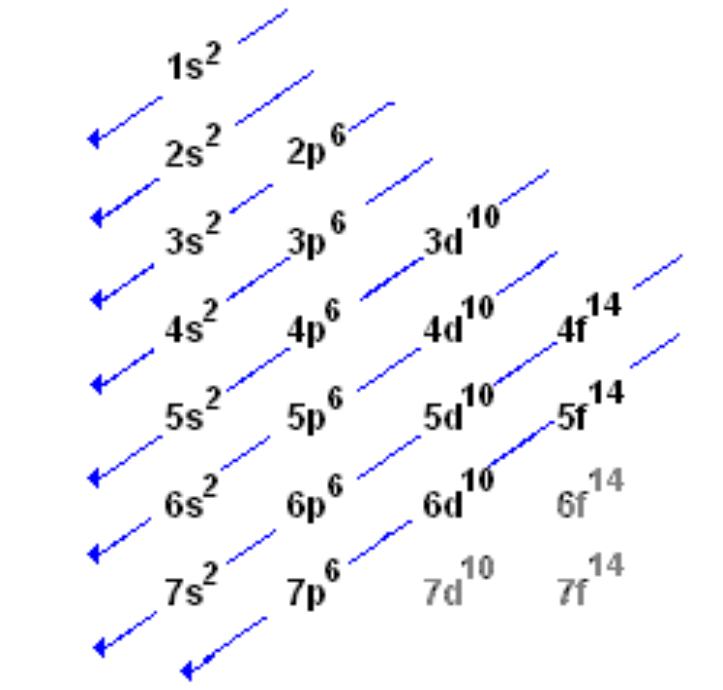
How to learn electron configuration. #protons=#electrons so if you have, let's say, 9 protons (fluorine). Using these rules, the electron configuration of any element can be theorized. So the electron configuration of sulfur will be 1s 2 2s 2 2p 6 3s 2 3p 4.
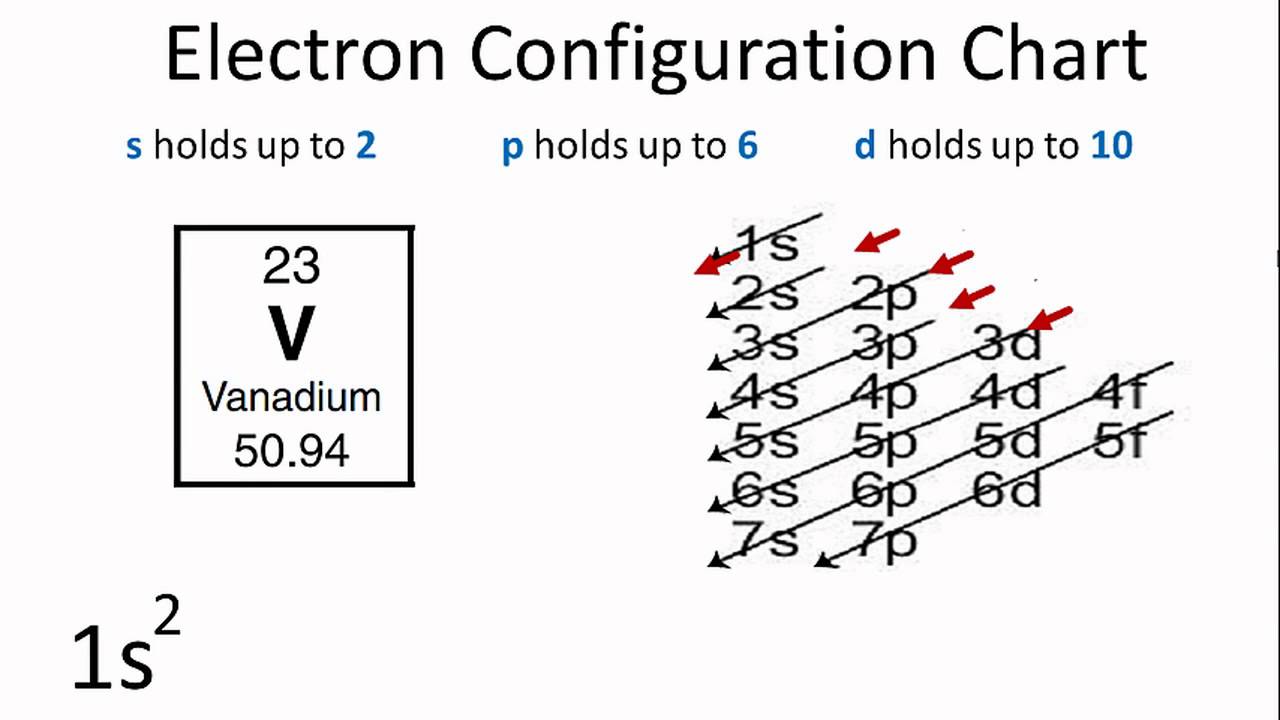
Get the atomic number of the element (# of protons) and fill each orbital (s, p, d, f) with electrons. 119 rows shorthand electron configuration full electron configuration electron shell arrangement; It is written out, as opposed to orbital diagrams which are depicted pictorially.
Electron configurations describe where electrons are located around the nucleus of an atom. It contains plenty of practice problems and examples including the. Electron configuration of hydrogen (h) 1s 1:
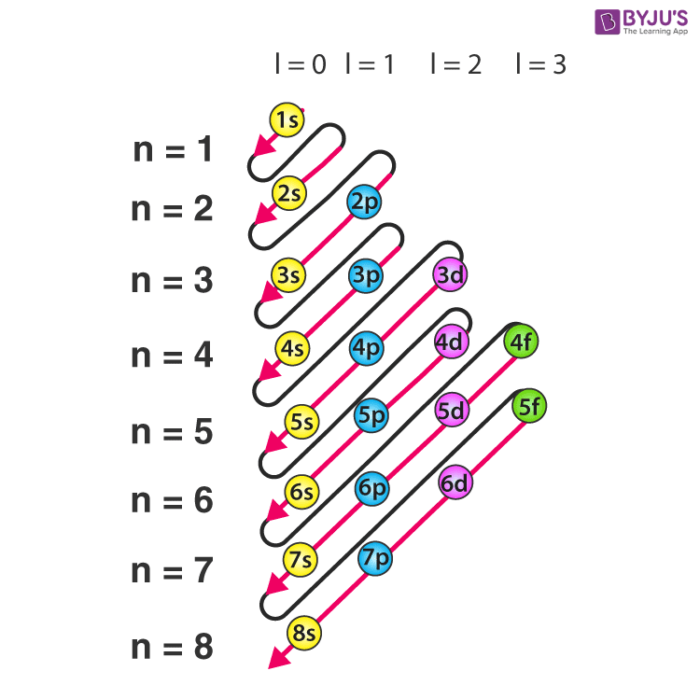
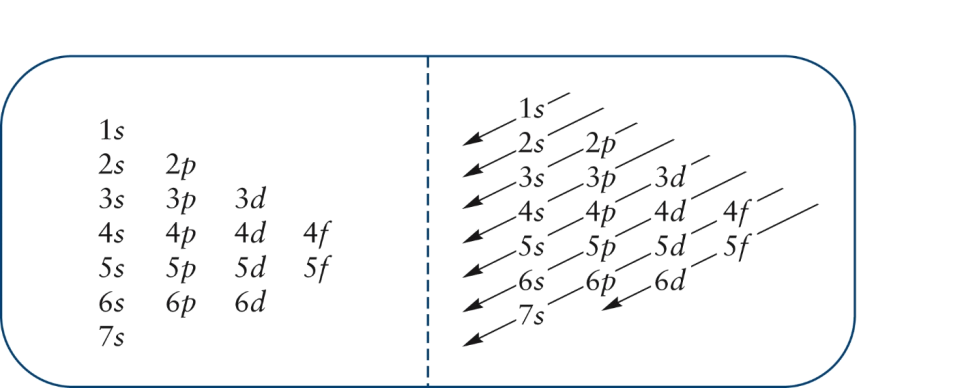
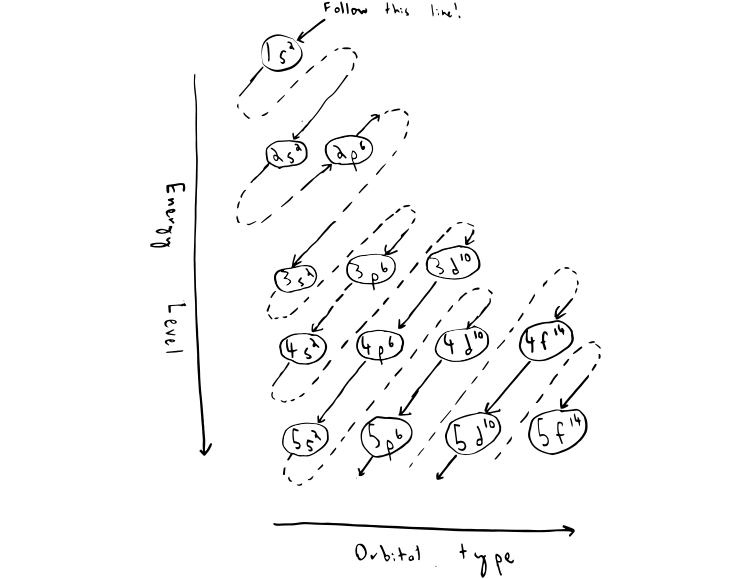
Each subshell has one or more. Start writing electron configuration from the very first element (i.e., hydrogen) all the way up to sulfur. Electron configuration is shorthand for the arrangement of electrons in atomic orbitals.
Each shell has one or more subshells within it. In the diagram above the energy levels are. There are electrons in all three p sublevels instead of.
The rule to calculate the number of electrons that each shell can hold is 2n 2. An up arrow and a down arrow have different “spins.”. First you should write their normal electron configuration and then when you remove electrons you have to take them from the outermost shell.